A Landmark Decision: CRISPR Gene Editing Therapy Approved for Sickle Cell Disease in England
The National Institute for Health and Care Excellence (NICE), the health watchdog in England, has delivered groundbreaking news for individuals battling severe sickle cell disease. In a momentous decision, NICE has approved the use of exagamglogene autotemcel (exa-cel), a CRISPR-based gene editing therapy, for eligible patients within the National Health Service (NHS). This landmark approval marks a significant advancement in the treatment landscape for sickle cell disease, offering a potential cure for a condition that has historically been managed with limited and often debilitating treatment options.
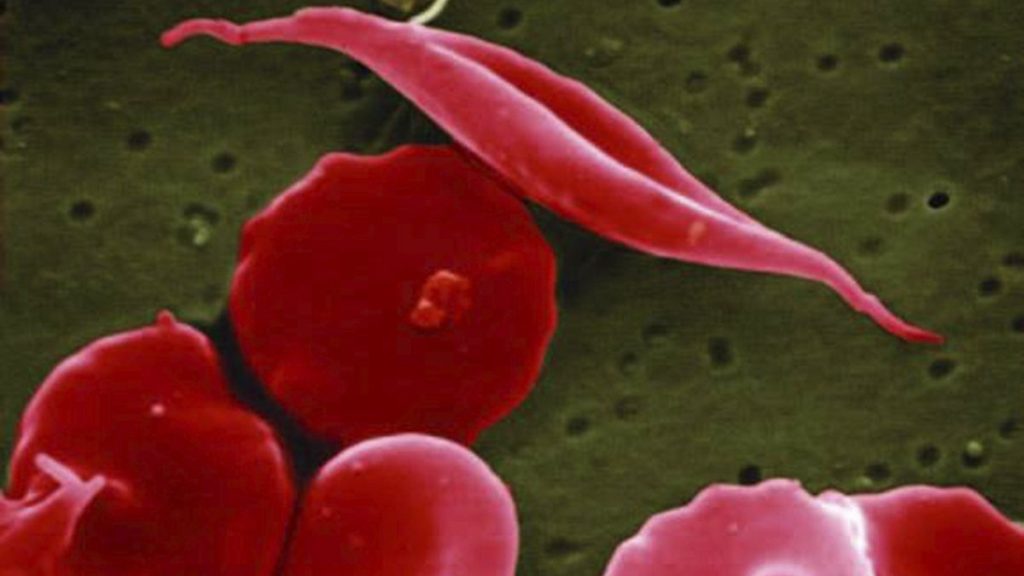
Sickle cell disease, a group of inherited blood disorders, primarily affects individuals of African, Caribbean, Middle Eastern, or South Asian descent. Characterized by abnormally shaped red blood cells, the disease leads to a cascade of debilitating symptoms, including severe pain crises, infections, anemia, and organ damage. Current treatments for sickle cell disease are often inadequate, providing only symptomatic relief and carrying the burden of significant side effects. Exa-cel offers a new paradigm in treatment, targeting the underlying genetic defect responsible for the disease.
The approval of exa-cel represents a beacon of hope for those living with the severe complications of sickle cell disease. The therapy, which carries a list price of £1.6 million (€1.9 million) per course, will be available to patients aged 12 and older who experience severe complications and for whom a stem cell transplant is deemed suitable but a matching donor cannot be found. This targeted approach addresses a critical unmet need for these patients, offering the potential for long-term remission and a significantly improved quality of life.
The decision by NICE to approve exa-cel comes after a period of careful evaluation. In earlier draft guidance, NICE had initially rejected the therapy for sickle cell disease, raising concerns about its cost-effectiveness. However, subsequent reviews and negotiations with the manufacturer, Vertex Pharmaceuticals, have led to a revised agreement that makes the therapy accessible to eligible patients within the NHS. This outcome underscores the complex considerations involved in evaluating and approving novel therapies, balancing clinical efficacy with affordability and accessibility.
Exa-cel, also known as Casgevy, is a revolutionary treatment that harnesses the power of CRISPR/Cas9 gene editing technology. The therapy involves extracting a patient’s blood stem cells, modifying them in a laboratory setting using CRISPR to correct the genetic defect responsible for sickle cell disease, and then reinfusing the edited cells back into the patient. This precise genetic modification aims to restore the production of healthy red blood cells, effectively addressing the root cause of the disease. The discovery of CRISPR/Cas9 technology was recognized with the Nobel Prize in Chemistry in 2020, highlighting its transformative potential in medicine.
While the approval of exa-cel is undeniably a cause for celebration, it’s essential to acknowledge that the therapy is not a panacea for all sickle cell disease patients. Its eligibility criteria restrict its use to a specific subset of patients with severe complications. Furthermore, long-term data on the effectiveness and safety of exa-cel are still being gathered. NICE plans to continue monitoring the therapy’s performance in real-world settings, collecting data from treated patients to inform future evaluations and ensure its continued safety and efficacy. Research efforts must continue to develop treatments that benefit a broader range of sickle cell disease patients and address the remaining challenges in their care.