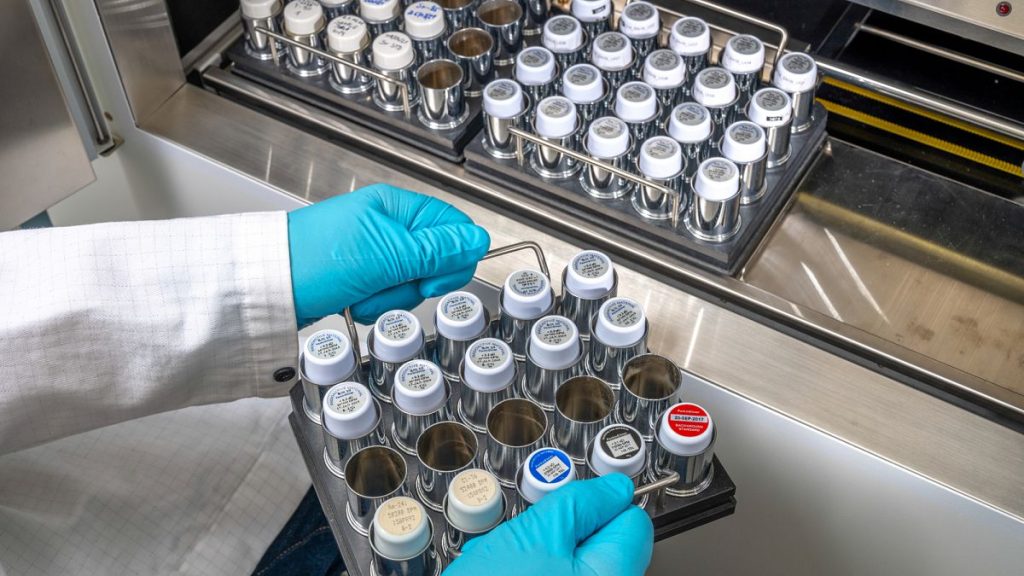
ESSAY: THE EU’S RELYANCE ON RUSSIA AND THE US FOR relie On radiopharmaceuticals across Europe, production alone isn’t enough to secure supply; this sector remains a critical dependency on geopolitical and supply bottlenecks. The European Medicines Agency (EMA) and the Heads of Medicine realize this and urge the EU to enhance its supply chain, tackling vulnerabilities beyond production. Despite steady demand, the EU is grappling with limited manufacturing capacity, fragmented transport regulations, and heavy reliance on third countries for raw materials, both in Russia and the United States.
radiopharmaceuticals vary across Europe depending on their medical roles, though iodine-123 is widely used for imaging and thyroid cancer treatment. They’re essential in diagnosing and treating conditions like tumors and have revolutionized healthcare but pose significant challenges due to their radioactive nature, which affects production and transport. The EMA has issued recommendations calling for stronger supply chain initiatives to address production and transport vulnerabilities. These include targeting production, transportation, and logistics issues, ensuring efficient flow through EU and cross-border networks.
Specific examples include Lead-130, a specialized fuel for medical isotope reactors. Russia remains the only commercial supplier capable of producing large quantities, through its state-owned company Tenex. The U.S., however, has ramping up production of HALEU within the last year, as the industry faced operational challenges over decades. Additionally, French nuclear fuel cosyure Orano is exploring plans to build a U.S. enrichment facility to lower Washington’s reliance on Russian raw materials.
The EMA’s recommendations aim to address these dependencies through several efforts. These include developing EU/HANDLEU enrichment capabilities, investing in transport solutions, and supporting initiatives like the European Radioisotope Valley Initiative and the Strategic Election for Medical Applications of Ionising Radiation (SAMIRA). Mapping their supply chains and developing strategies aligned with a broader EU framework are key priorities.
Transportation bottlenecks and lead restrictions are overshadowing demand. Both industrial and regulatory barriers impede efficient delivery of radiopharmaceuticals, which are critical for timely treatments. The EMA suggests that transport container certification standards and mutual recognition among EUP member states are essential to overcoming these challenges. Without such agreements, delays and inefficiencies could escalate.
The EU has already banned lead in shot, but restrictions on lead in containers are being considered. This aligns with concerns about health sovereignty and environmental impact. The EMA emphasizes that acknowledge the potential consequences of such bans in case scenarios, providing a presumption of adaptability and resilience in the industry.
**In summary, the EU’s reliance on Russia and the U.S. for medical radiopharmaceuticals exists, but efforts, including strengthening the supply chain and ensuring transport efficiency, are critical to achieving a sustainable and reliable health care future.